







COVID-19 Test Home Collection Kit DTC
$109
Free Shipping - FSA / HSA Eligible - Ages 18+
Easily test for SARS-CoV-2 infection whether you’re symptomatic or asymptomatic. Collect your sample at home and ship it free for secure digital results within 24-48 hours of the lab receiving your sample. A telehealth consult is available to guide you through your next steps.
RNA from SARS-CoV-2 virus
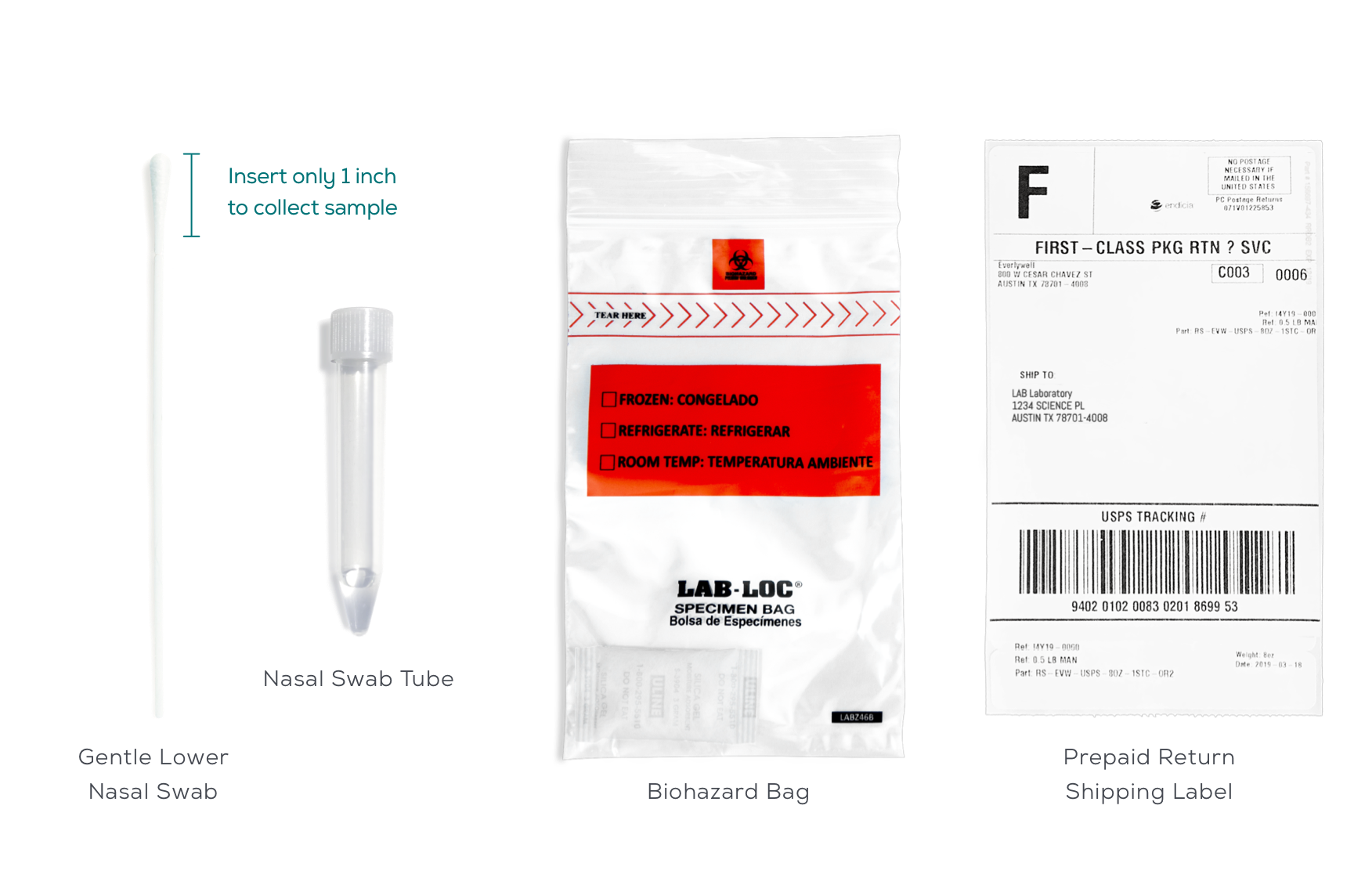
Gentle lower nasal swab for easy self-collection
Find out whether you are infected with SARS-CoV-2









COVID-19 Test Home Collection Kit DTC
Easily test for SARS-CoV-2 infection whether you’re symptomatic or asymptomatic. Collect your sample at home and ship it free for secure digital results within 24-48 hours of the lab receiving your sample. A telehealth consult is available to guide you through your next steps.
RNA from SARS-CoV-2 virus
Gentle lower nasal swab for easy self-collection
$109
Free Shipping - FSA / HSA Eligible - Ages 18+
Questions?
The Everlywell COVID-19 Test Home Collection Kit DTC has received an EUA from the FDA and is used with EUA authorized RT-PCR tests, which is the preferred COVID-19 testing method for many travel requirements. Many destinations require negative results within a very narrow timeframe. Included in your digital results will be a printable version that you can share. We recommend that you reach out to your destination’s government for their specific requirements and timelines before making your purchase.
Included in your digital results will be a printable version that you can share. You can find many answers to common questions about the collection kit and testing here.
While we are not able to provide an exact estimate of when you will receive your results, keep in mind the typical turnaround time for results. As a reminder:
- Order Delivery: we estimate that you will receive your order within 2-8 business days of placing your order, depending on the shipping method you choose. (You also have the option of purchasing expedited shipping to your home for an additional fee.)
- Returning Sample to Lab: once you ship your sample at a UPS dropbox Monday-Friday, our partner lab should receive within 1-2 business days.
- Results: results are typically available within 24-48 hours after the lab receives your sample.
If you are concerned that your schedule is too tight to allow for unforeseen delays, we recommend that you check your state health department's website for other testing options.
You can find the latest country-specific requirements for international travel at travel.state.gov.
The samples collected using the Everlywell COVID-19 Test Home Collection Kit DTC are meant for RT-PCR tests (a specific kind of molecular PCR test) that look for the presence or absence of unique genomic markers of the SARS-CoV-2 virus using a nasal swab for sample collection.
To date, the FDA has not observed evidence to suggest the sensitivity of the tests used by our partner labs are impacted by known circulating variants. All analyses conducted to date indicate that our full range of COVID-19 diagnostic offerings will accurately detect SARS-CoV-2 infection caused by Omicron or other variants of concern (VOC), and we will continue to carefully monitor for any changes in test performance.
Our partner labs are taking proactive actions to confirm the existing tests are robust to the detection of these known new variants. These confirmation studies indicate the current tests do detect the virus, even with the new variants. As more information regarding the variants becomes available, further confirmation and potential optimization steps will be taken.
Once your order is complete, your package will be shipped from our fulfillment warehouse within 1-2 business days (Monday - Friday). We estimate that you will receive your package within 2-8 business days, depending on the shipping method you choose. You also have the option of purchasing expedited shipping to your home for an additional fee. Kits that are ordered Friday through Sunday will not be shipped until the following week.
In order to get your sample to our labs as quickly as possible, we provide a prepaid UPS shipping label. Samples should be returned to a UPS dropbox Monday-Friday. Results are typically available within 24-48 hours after the lab receives your sample. All labs processing COVID-19 tests are operating Monday-Saturday. You will receive an email when your results are ready. You can find more information about the process here.
While we have done everything we can to make the process as quick and easy as possible, please keep in mind that shipping times have been unpredictable recently (you can find more about this on the USPS and UPS websites).
If you are concerned that your schedule is too tight to allow for unforeseen delays, we recommend that you check your state health department's website for other testing options.
You can find the latest country-specific requirements for international travel at travel.state.gov.
Like our other products, the Everlywell COVID-19 Test Home Collection Kit DTC will come with everything you need to collect a sample at home (generally in five minutes or less) and safely ship your sample to a CLIA-certified laboratory.
You will use the provided materials to collect a lower nasal sample using a swab. The swab tip does not need to be inserted far—only about an inch into the front part of your nostril. Please refer to our video and print instructions for more information.
In order to get your sample to our labs as quickly as possible, we provide a prepaid UPS shipping label. Samples must be collected and returned to a UPS drop box the same day, Monday through Friday, and before the carrier’s last pickup time for the day.
You can find drop box locations on the UPS website.
YOUR SAMPLE WILL BE REJECTED IF:
- The swab is not in the tube with the saline solution.
- The tube is not sealed and in the biohazard bag.
- The kit is not registered or if the ID sticker is not filled out and affixed to your sample tube.
- The sample is mailed on Saturday or Sunday, or after the last pickup of the day. Do not deliver your test sample to a drop box on the weekend.
- The sample is scheduled for UPS pickup or is dropped off at a store location.
- The sample expires before the lab is able to process it (5-7 days from collection).
Results are typically available within 24-48 hours after the lab begins processing the sample. All labs processing COVID-19 tests are operating Monday-Saturday. You will receive an email when your results are ready.
You can find more information on our blog as well as safety tips for COVID-19 prevention and treatment here.
You should receive emails confirming your order, shipment to you (with tracking number), delivery to you, registration, delivery to the lab (with tracking number), and a final email when your results are ready. Once your kit has been registered, you should also be able to track processing at the lab on your dashboard. However, due to the increase in volume and rapid turnaround time, email notifications and dashboard updates may be delayed.
We recommend making a note of your tracking number on the shipping label so you can track your sample through the UPS website. If you have questions about your shipping status, please reach out to:
Everlywell offers a rapid antigen test for COVID-19 (also referred to as a rapid COVID-19 test):
The BD Veritor™ At-Home COVID-19 is an Emergency Use Authorized COVID-19 rapid antigen test available for purchase through Everlywell.The serial test is intended to be used twice over two to three days, with at least 24 hours and no more than 48 hours between tests. The test enables you to collect your sample at home and receive digital results displayed on your smartphone through the Scanwell Health App in just 15 minutes. This test is authorized for anyone aged 2+ (ages 2-13 require adult-assisted collection whereas ages 14+ can self-collect). Individuals under 18 years of age will need a parent/guardian to agree to the terms of use within the Scanwell® Health App.
For individuals looking for COVID-19 PCR testing, our FDA-authorized COVID-19 Test Home Collection Kit DTC may be appropriate. A prepaid UPS shipping label is provided to get the sample to the lab as quickly as possible, and results for this test are typically available within 24-48 hours after the lab begins processing the sample.
People with COVID-19 have had a wide range of symptoms reported – ranging from mild symptoms to severe illness. Some people do not show symptoms at all.
Symptoms may appear 2-14 days after exposure to the virus. People with these symptoms may have COVID-19:
- Cough
- Shortness of breath or difficulty breathing
- Fever
- Chills
- Muscle pain
- Sore throat
- New loss of taste or smell
This list is not all possible symptoms. Other less common symptoms have been reported, including gastrointestinal symptoms like nausea, vomiting, or diarrhea.
Anyone who thinks they may have been exposed to COVID-19, or those who are currently experiencing symptoms of COVID-19 such fever, cough, or shortness of breath. Those who are not experiencing symptoms are still eligible for this test, as it is possible to be infected by SARS-CoV-2 without being symptomatic.
You will be asked to complete a health intake survey when you register your test during at-home sample collection. If your symptoms are severe, such as difficulty breathing, it will be recommended that you prioritize seeking emergency medical attention right away and do not take this test. The CDC recommends calling your healthcare provider or facility to let them know that you may have COVID-19 which will help them to take steps to keep others from being exposed.
If you’re experiencing severe symptoms, such as difficulty breathing, seek medical attention right away. The CDC recommends calling your healthcare provider or facility to let them know that you may have COVID-19 which will help them to take steps to keep others from being exposed.
At this time we are only able to offer coronavirus testing for those 18 years and older. Please see our FAQ here.
This test may not be helpful if you:
- Currently have severe symptoms that limit your daily activities. Seek medical attention right away if this is the case.
Note: If you are a healthcare professional, consult your place of work for guidance about testing, whether you should stay home, and when you should return to work. You should follow the testing recommendations set forth by your employer as they may differ from the CDC’s guidelines.
Yes, the Everlywell COVID-19 Test Home Collection Kit DTC and our lab partners have received Emergency Use Authorization from the FDA.
This home collection kit has not been FDA cleared or approved. This home collection kit has been authorized by FDA under an EUA. This home collection kit has been authorized only for the home collection and maintenance of nasal swab specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This home collection kit in combination with the authorized test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Everlywell provides detailed instructions in our kit and online for safe collection and transport of samples. We provide the appropriate shipping materials to safely ship samples to our partner labs following the recommendations provided by the United Nations Committee of Experts on the Transport of Dangerous Goods (UN).
We recommend that our customers take precautionary measures when collecting their sample by washing their hands thoroughly, disinfecting surfaces where they will be completing their test and using the provided alcohol prep pad to disinfect the shipping sleeve before placing your sample in the mail (though be sure to not wipe the shipping label which could impact legibility).
Everlywell is not a healthcare provider nor a laboratory and therefore cannot bill your health insurance directly. However, it is required by federal law that your insurance provider covers this test. In addition, this product is covered by participating FSA and HSA plans. The test cost is transparent, paid up-front and we will never send you a surprise bill.
Once your test is completed, Everlywell will provide an itemized receipt that will contain the information you will need to apply for reimbursement directly from your health insurance provider. We always recommend checking with your benefits provider about coverage, especially if you are purchasing multiple tests. Learn more about reimbursement and read the federal guidelines here.
- What does the COVID-19 Test Home Collection Kit measure?
- How quickly will I get my COVID-19 Test Home Collection Kit and results?
- Can I use my COVID-19 Test Home Collection Kit results for travel purposes?
- How do I collect my COVID-19 Test Home Collection Kit sample from home?
- How can I track the status of my COVID-19 Test Home Collection Kit order and results?
- Additional COVID-19 Test Home Collection Kits FAQs
- General Coronavirus Information
- COVID-19 Test for Healthcare Providers
Would you like to order this test?
Shipping Address
,