
130+ Coronavirus Statistics You Might Not Know in 2022
Last updated: March 3, 2022
Coronavirus disease 2019 (COVID-19) is a respiratory illness that spreads among people in close contact, primarily through droplets produced when an infected person coughs or sneezes. It can carry with it symptoms of fever, cough, fatigue, and shortness of breath, among others.
The disease — declared a pandemic by the World Health Organization on March 11, 2020 — has taken a toll on schools, workplaces, small businesses, and healthcare facilities across the globe. With vaccines — namely Moderna, Pfizer-BioNTech, and Johnson & Johnson/Janssen — authorized by the FDA beginning in December 2020, many people around the world have been immunized against the virus, but there is still risk of transmission through vaccinated people.
Here are some key statistics to know about COVID-19.
- Background
- Transmission
- Symptoms
- Testing
- Survival Rate by Age
- Coronavirus vs. the Flu
- Community Response
- Vaccinations
- Breakthrough Cases
- Variants
1. Coronavirus Background
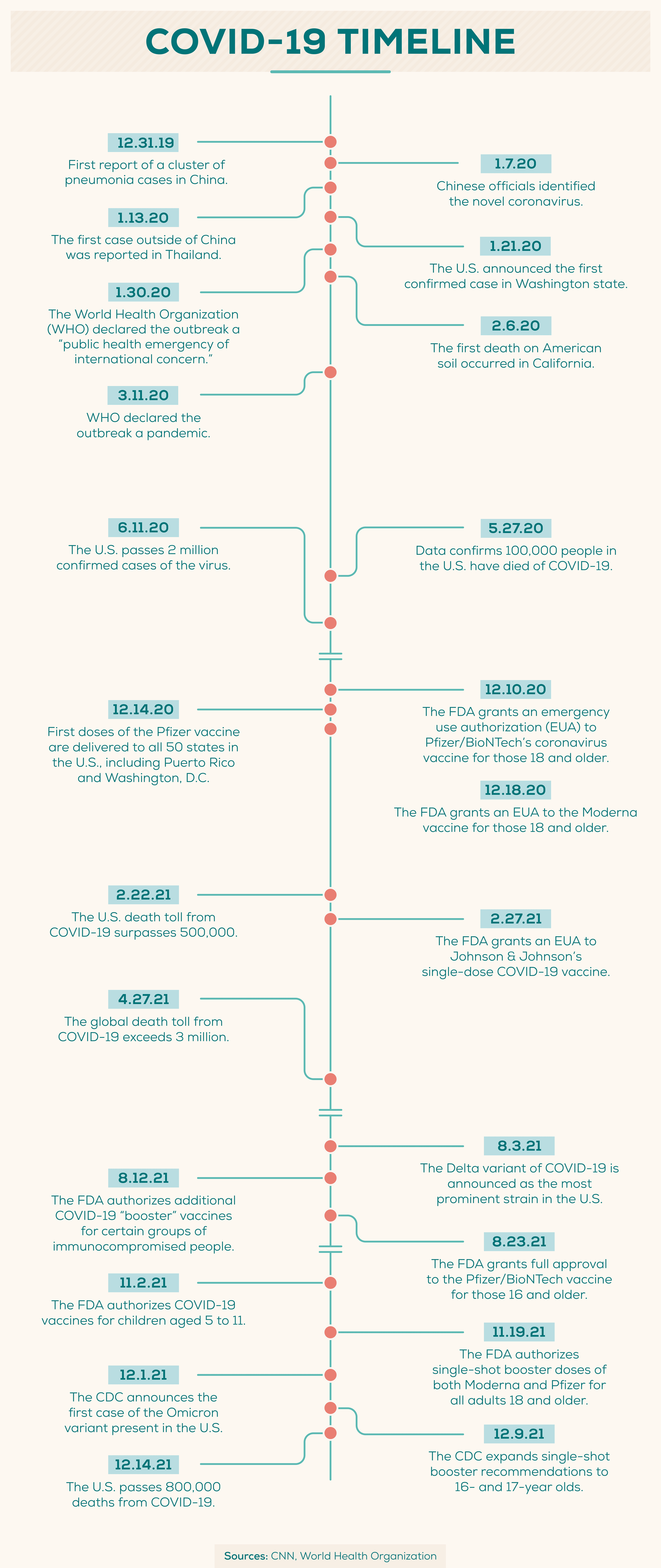
In December 2019, health officials identified several similar cases of pneumonia in Wuhan, the capital of China’s Hubei province. Chinese health officials identified the cause of the pneumonia as a novel (or new) coronavirus, which was later named SARS-CoV-2.
The virus itself is known as SARS-CoV-2, or Severe Acute Respiratory Syndrome Coronavirus 2, while the disease that results from the virus is called coronavirus disease 2019, or COVID-19.
- Coronaviruses are a large family of viruses that are common in people and animals, such as camels, cattle, cats, and bats. (NIH)
- Other coronaviruses include MERS-CoV, which was responsible for Middle East Respiratory Syndrome (MERS) first reported in Saudi Arabia in 2012, and SARS-CoV, which was responsible for Severe Acute Respiratory Syndrome (SARS) first reported in Asia in February 2003. (CDC)
- SARS-CoV-2, like MERS-CoV and SARS-CoV, most likely originated in bats. (Nature Communications)
- Four types of human coronaviruses found around the world account for 10–30% of upper respiratory tract infections in adults. (Journal of the American Medical Association)
- The World Health Organization declared the outbreak a “public health emergency of international concern” on January 30, 2020, and a pandemic on March 11, 2020. (WHO and WHO)
2. Coronavirus Transmission
According to the Centers for Disease Control and Prevention, the virus is thought to spread mainly among people in close contact with one another (six feet or less) through droplets produced when an infected person coughs or sneezes. The droplets can land in other people’s mouths or noses or be inhaled.
The virus may also spread when people touch contaminated surfaces and later touch their mouths, noses, or eyes.
- There are three main ways that COVID-19 is thought to spread: (CDC)
- Inhaling infected droplets or particles when close to an infected person.
- Having those infected droplets or particles land on the eyes, mouth, or nose.
- Touching the eyes, nose, or mouth with hands that have been infected.
- On average, each infected person spreads the infection to another two to three people. (Nature)
- Large droplets of the coronavirus may last the air for seconds to minutes, while smaller droplets may linger for minutes to hours. (CDC)
- Certain high-percentage copper alloy products can eliminate 99.9 percent of SARS-CoV-2 within two hours. (EPA)
- On nonporous surfaces such as steel and glass, a 99 percent reduction in coronaviruses can be expected within three days in typical environments. (CDC)
- Human coronaviruses on surfaces can be inactivated within a minute using disinfectants with 62–71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite. (Journal of Hospital Infection)
- Viruses similar to the strain that causes COVID-19 can survive on clothing and transmit to other surfaces for up to 72 hours. (De Montfort University Leicester)
- As of February 2021, there is no evidence that suggests COVID-19 can spread to humans through the use of recreational waters, such as swimming pools or hot tubs. (CDC)
3. COVID-19 Symptoms
Research suggests that people are most contagious when they show the most symptoms, but it may be possible for someone who doesn’t show any symptoms to spread the virus (otherwise known as an asymptomatic infection).
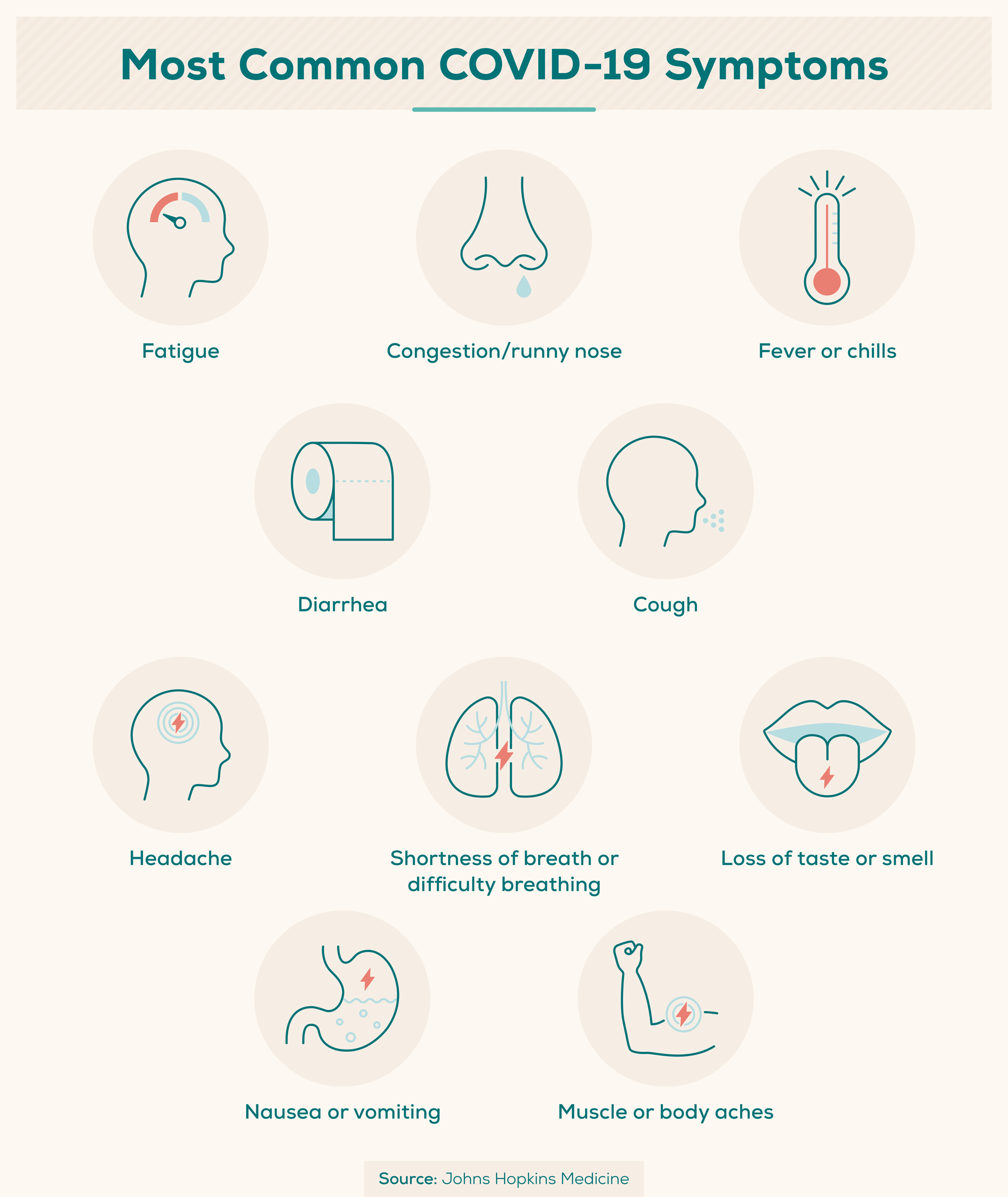
If you are experiencing common symptoms of COVID-19 (such as a dry cough, fatigue, muscle or body aches, or shortness of breath) you may want to consider getting tested, especially if you were previously around someone who has tested positive.
If you are experiencing severe COVID-19 symptoms (such as pain or pressure in the chest and difficulty breathing) you should call 911 immediately.
- COVID-19 symptoms may appear two to 14 days after exposure. (CDC)
- There is a mean incubation period of five days. (CDC)
- COVID-19 symptoms may last about two weeks in mild cases and three to six weeks in severe or critical cases. (WHO)
Generally, common symptoms of COVID-19 include: (Johns Hopkins Medicine)
- Congestion/runny nose
- Cough
- Diarrhea
- Fatigue
- Fever or chills
- Headache
- Loss of taste or smell
- Muscle or body aches
- Nausea or vomiting
- Shortness of breath or difficulty breathing
Symptoms of severe COVID-19 that warrant calling the hospital include: (Johns Hopkins Medicine)
- Bluish lips or face
- Confusion
- Difficult breathing
- Inability to wake up or stay awake
- Persistent pain or pressure in the chest
4. COVID-19 Testing
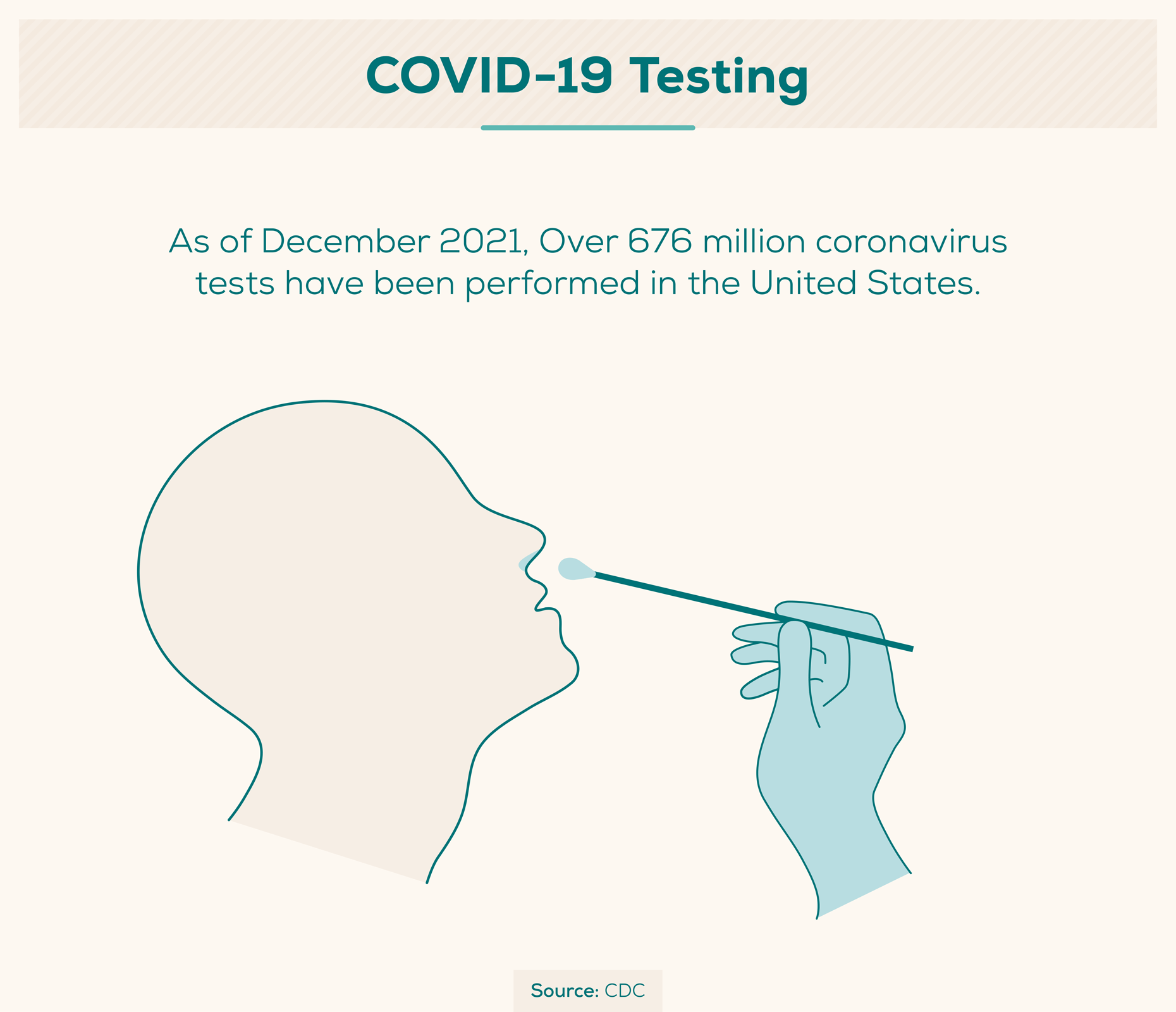
Health experts have stressed the need to test as many people as possible so that those with COVID-19 know if they have it and can take appropriate action. Throughout the pandemic, it has remained especially important for frontline healthcare workers to have access to testing since they not only play a critical role in caring for sick patients, but also face a high risk of exposure to COVID-19.
Everlywell has partnered with hospitals, clinics, nursing homes, and government agencies to supply testing to thousands of healthcare workers, seniors, and high-risk patients.
- On March 4, 2020 — a week after the first confirmed community transmission case in the U.S. — the U.S. had tested fewer than 1,000 people total. (The COVID Tracking Project)
- At that same point (March 4, 2020), South Korea, which was quick to mobilize testing, had tested almost 150,000 people and was testing more than 10,000 people per day. (KCDC)
- Over 761 million coronavirus tests have been reported in the United States as of January 2022. (CDC)
- The seven-day positivity rate is 26.15% as of January 2022. (CDC)
- The United Kingdom ranks highest for the rate of coronavirus tests performed per million population. (Statista)
- Coronavirus tests typically swab the back of the nose. (Everlywell)
- The Everlywell COVID-19 Test initially launched with 30,000 kits, with the kits going to those who needed them the most: healthcare workers, seniors, and high-risk patients. (Everlywell)
5. COVID-19 Fatality Statistics
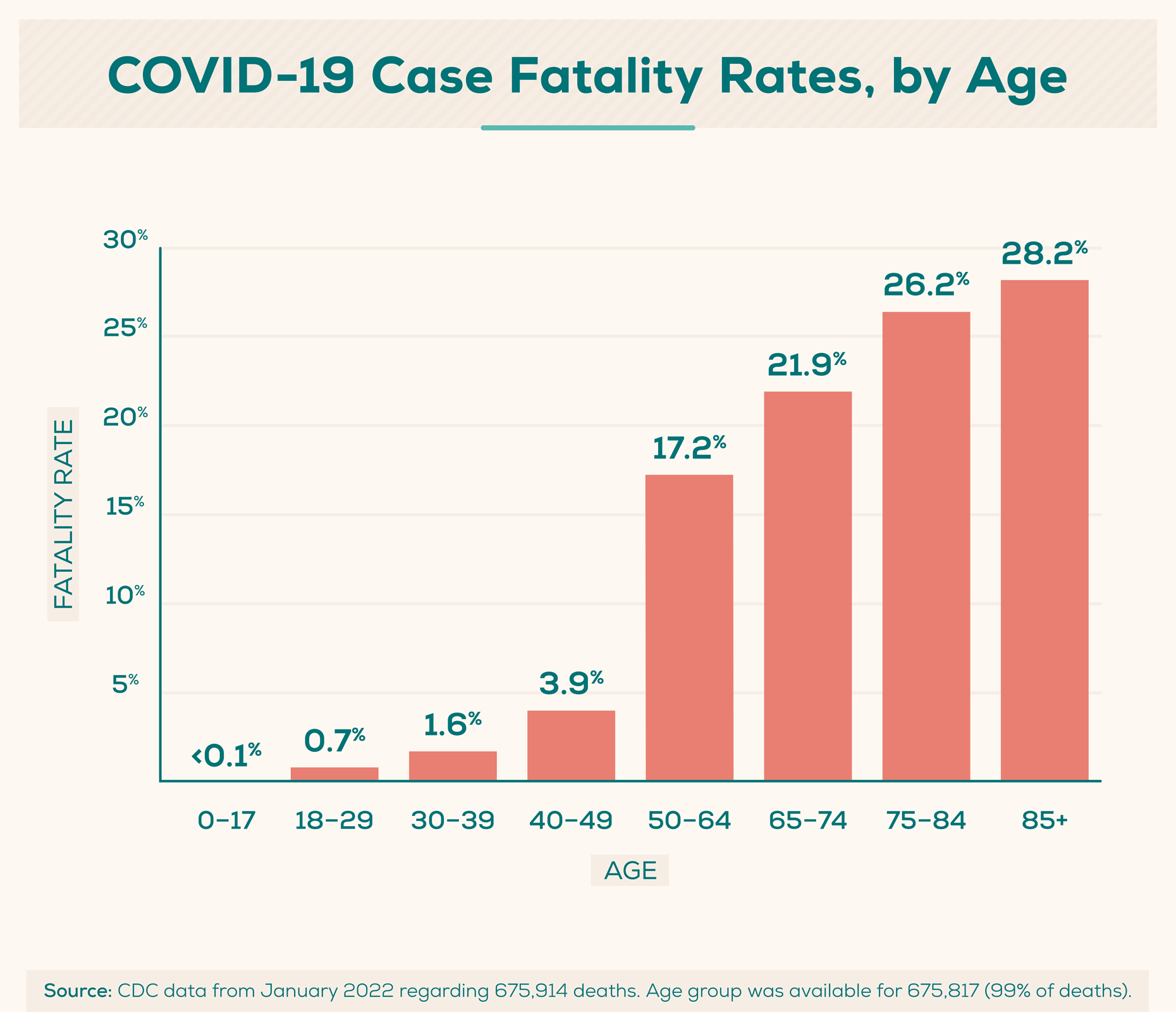
Researchers often look at the case fatality rate, which is the proportion of people who die from a disease of those who have been diagnosed with it. The case fatality rate can vary substantially over time depending on the specific characteristics of those infected.
- As of January 2022, there have been over 858,000 deaths in the U.S. related to COVID-19. (CDC)
- The highest number of deaths attributed to COVID-19 in the U.S. have occurred in the 75+ age group. (CDC)
- The highest number of reported cases in the U.S. have been by those in the 18–29-year-old age group. (CDC)
- More men than women have died of the virus, though more women than men have reported total cases. (CDC)
Deaths from COVID-19 in the U.S. are broken down as follows by age as of January 2022: (CDC)
- Ages 0–4: 0% of total deaths
- Ages 5–11: 0%
- 12–15: 0%
- 16–17: 0%
- 18–29: 0.8%
- 30–39: 1.8%
- 40–49: 4%
- 50–64: 17.6%
- 65–74: 22.1%
- 75–84: 26%
- 85+: 27.7%
Total fatalities based on race/ethnicity, including all age groups, in the U.S. are as follows as of January 2022: (CDC)
- American Indian/Alaska Native, Non-Hispanic: 1.1% of total deaths
- Asian, Non-Hispanic: 3.4%
- Black, Non-Hispanic: 13.4%
- Hispanic/Latino: 16.9%
- Multiple/Other, Non-Hispanic: 2.4%
- Native Hawaiian/Other Pacific Islander, Non-Hispanic: 0.2%
- White, Non-Hispanic: 62.5%
6. COVID-19 vs. the Flu
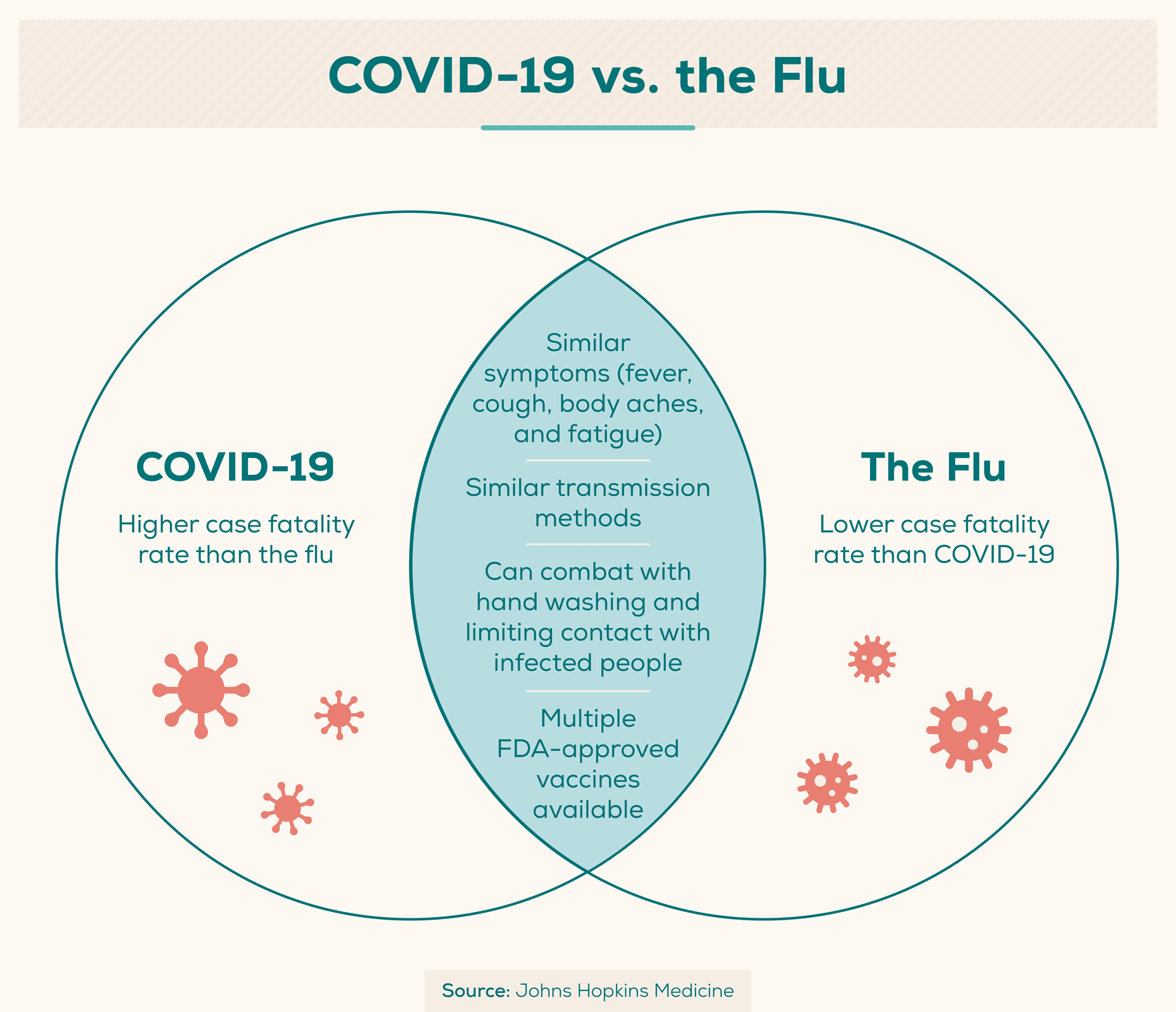
COVID-19 and influenza, or “the flu,” are both infectious respiratory illnesses that may carry many of the same symptoms: fever, cough, and fatigue. Both may be spread via the same methods and can be prevented by thorough hand-washing and social distancing. Below are some key differences and similarities between COVID-19 and the flu.
- COVID-19 can sometimes cause a person to lose their sense of taste (ageusia) or smell (anosmia), which very rarely happens with the flu. (Johns Hopkins Medicine)
- One or more days can pass between infection and showing symptoms for both COVID-19 and the flu. (CDC)
- Typically, a person will experience flu symptoms one to four days after infection.
- COVID-19 symptoms are typically experienced five days post-infection, but can appear anywhere from two to 14 days after infection.
- One may spread both the flu and COVID-19 virus for at least one day before experiencing symptoms. (CDC)
- COVID-19 appears to cause more serious illnesses than the flu. (CDC)
- Multiple FDA-approved vaccines are produced annually to protect against the flu, and three COVID-19 vaccines have been authorized under an EUA by the FDA. (CDC)
7. Community Response
The philanthropic response to COVID-19 has been enormous, with organizations and individuals pitching in billions of dollars to help those affected by the pandemic.
- $27.3 billion in pledges and grants have been earmarked for the coronavirus outbreak, as of January 2022. (Candid)
Philanthropy spent over $20 billion on COVID-19 in 2020: (Candid + CDP)
-
$9 billion+ from corporations/corporate foundations
-
$5 billion+ from high-net-worth individuals
-
$4 billion+ from independent foundations
-
$1 billion+ from public charities, community foundations, or operating foundations
-
Over 72,700 grants and pledges have been made as of January 2022 to fund coronavirus research or relief. (Candid)
-
Everlywell funded a $1 million Development Incentive Program to increase capacity for COVID-19 testing. (Everlywell)
-
Everlywell has provided thousands of tests to frontline healthcare workers, seniors, and high-risk patients nationwide. (Everlywell)
8. Vaccinations
Starting in December 2020, the Food and Drug Administration (FDA) authorized emergency use in the United States of the Pfizer-BioNTech COVID-19 vaccine, followed by Moderna and Johnson & Johnson’s Janssen vaccine. Moderna and Pfizer-BioNTech vaccines are administered in two doses several weeks apart, while Johnson & Johnson vaccines are received in a single dose.
For the purpose of these statistics, “fully vaccinated” refers to those who received either two doses of Moderna or Pfizer, or one full dose of Johnson & Johnson. People aged 16 and older are now eligible to receive additional doses, or vaccine booster shots, at least six months after receiving the primary series. The CDC has more information on vaccine booster eligibility and guidelines.
Globally, there are a number of vaccines available in addition to the three U.S.-based vaccines. Oxford/AstraZeneca, Sinovac, Sputnik V, and Sinopharm/Beijing, to name a few, are some additional vaccines available outside of the U.S.
- It’s advised that everyone age 12 and older gets vaccinated as soon as possible. (CDC)
- As of January 2022, over 532 million vaccine doses have been administered in the U.S. (Our World in Data)
- In the U.S., 75.3% of the population has received at least one dose of the COVID vaccine as of January 2022. (CDC)
- 63.2% of the U.S. population has been fully vaccinated against COVID-19 as of January 2022. (CDC)
- Globally, over 37.34 million COVID-19 vaccines are administered daily. (Our World in Data)
- Over 52% of the global population have received at least one dose of a COVID-19 vaccine. (Our World in Data)
- Over 9.8 billion doses of coronavirus vaccines have been administered globally. (Our World in Data)
By age group, vaccine rates in the U.S. are as follows as of January 2022: (USAFacts)
At least one dose:
- 75 years or older: 99.22%
- 65–74: 100%
- 50–64: 91.67%
- 40–49: 84.69%
- 25–39: 77.24%
- 18–24: 74.29%
- 12–17: 65.18%
- 5–11: 28.12%
Fully vaccinated:
- 75 years or older: 84.81%
- 65–74: 90.34%
- 50–64: 79.03%
- 40–49: 72.16%
- 25–39: 64.01%
- 18–24: 59.92%
- 12–17: 54.62%
- 5–11: 18.79%
9. Breakthrough Cases
Though being vaccinated against COVID-19 lessens the risk of being hospitalized after contracting the virus, it does not entirely protect against catching the virus. Testing positive for COVID-19 despite being vaccinated is known as a “breakthrough case,” and the CDC notes that they are to be expected, as with any other vaccine, even if you have received a booster vaccine.
- COVID-19 vaccines are effective at granting immunity and preventing infection from the virus, but like other vaccines, are not 100% effective at preventing infection. (CDC)
- Vaccinated people who experience a breakthrough case are less likely to experience serious symptoms or develop a serious illness than an unvaccinated person. (CDC)
- Unvaccinated people are six times more at risk to test positive for the virus compared to fully vaccinated people. (The White House)
- Compared to fully vaccinated people, unvaccinated people are 14 times more at risk to die after contracting COVID-19. (The White House)

- One in 250 fully vaccinated people test positive for COVID-19, while one in 82 unvaccinated people test positive. (Avera Health)
- For unvaccinated adults 18 to 49, hospitalization rates associated with COVID-19 are 12 times higher than for vaccinated adults. (CDC)
- Fully vaccinated people who experience breakthrough cases with the Delta variant can spread the virus to others, though they spread it for shorter periods of time than unvaccinated people. (CDC)
10. Variants
RNA diseases such as the coronavirus mutate over time, causing variants of the original virus that can be more contagious than the original. This is commonly seen in diseases like the flu, which is why doctors recommend getting a yearly flu shot.
In addition to the original strain of COVID-19 detected in Wuhan, China in December 2019, there have been five other “Variants of Concern” of the coronavirus that are unique from the original (World Health Organization).
- The Alpha variant was first documented in the United Kingdom in September 2020, and was designated as an official variant in December 2020.
- The Beta variant was first detected in South Africa in May 2020 and was designated in December 2020.
- The Delta variant was first recorded in India in October 2020 and was designated in April 2021.
- The Gamma variant was first documented in Brazil in November 2020 and was designated in January 2021.
- The Omicron variant was identified in multiple countries, including South Africa, in November 2021 and was designated the same month.
- As of January 2022, the two Variants of Concern present in the U.S. are the Delta and Omicron variants (CDC).
Two additional “Variants of Interest” have been identified that may affect virus characteristics such as disease severity or transmissibility, or cause significant community transmission. (World Health Organization)
- The first sample of the Lambda variant was recorded in Peru in December 2020 and was designated in June 2021.
- The Mu variant was first identified in Colombia in January 2021, and was designated in August 2021.
Delta Variant
- The Delta variant is more than two times as contagious as previously identified variants. (CDC)
- The Delta variant may cause more serious illness than other variants in people who have not been vaccinated. (CDC)
Omicron Variant
- Omicron may be spread more easily than the Delta variant, as well as additional variants. (CDC)
- Some monoclonal antibody treatments may not be as effective against Omicron infection, research suggests. (CDC)
- Due to the small number of cases as of January 2022, the severity of illness and risk of death associated with the Omicron variant is unclear. (CDC)
- Experts at the CDC predict that anyone exposed to the Omicron variant, despite vaccination stats or if they are showing symptoms, can spread the virus to others. (CDC)
- Vaccines should protect against severe illness, hospitalization, and death associated with the Omicron variant. (CDC)
- The CDC continues to recommend masks and vaccines as the best public health measures that protect against infection from all variants, including Omicron. (CDC)
If you’re experiencing symptoms such as fever, cough, or shortness of breath, you’ve been in close contact with someone confirmed or suspected to have COVID-19, you’ve been to certain countries where widespread community transmission has been reported, or you’ve been in community areas where COVID-19 cases have been reported, you should seek testing, even if you are partially or fully vaccinated.
Experiencing COVID-19 symptoms and looking for relief? Get COVID-19 treatment online via Everlywell's Virtual Care offering.
Disclaimer: This content was last updated on February 18, 2022 and some of the information provided may no longer be up to date. For the most recent information on COVID-19, visit the Centers for Disease Control and Prevention (CDC) website.